New Rule Reflects CMS Intention to Overhaul Meaningful Use Program
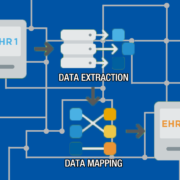
In an attempt to improve communications between providers and patients, promote interoperability through flexibility and make EHR access easier for patients, CMS released a new rule on April 24, 2018. This reflects a strong intention by the agency to overhaul the meaningful use program, in service under the name Medicare and Medicaid EHR Incentive Programs from its inception, including renaming the program “Promoting Interoperability”. But what does this mean for your orthopedic practice?
New Rule Reflects CMS Intention to Overhaul Meaningful Use Program
The program was started in 2011 to encourage the use of certified EHR systems by practitioners. Now that virtually all practices are using EHRs, CMS is changing direction by looking at changing priorities. The rule proposes changes to Medicare payment policies and rates under IPPS and LTCH PPS. The changes is planned to advance patient-driven healthcare systems by improving transparency and interoperability, reducing the burden on healthcare systems to improve flexibility while still providing patients easy access to healthcare records.
The changes are expected to start implementing core sections of the government-wide MyHealthEData initiative. It will happen through several steps to improve the sharing of healthcare information between providers. It’s expected that these changes will help create a more patient-centric approach to healthcare decisions, switching from the quantity of patients seen by a provider to the quality of care each patient receives.
The rule takes effect on October 1, 2018, and will impact approximately 3,300 acute-care hospitals and 420 long-term-care facilities. It reaffirms the requirement for provider use of the 2015 Edition of CEHRT in 2019 to demonstrate meaningful use to receive incentive payments and avoid Medicare payment reductions, including the use of APIs. For 2019 and 2020, reporting periods for both new and returning participants must be a minimum of a continuous 90-day period within each calendar year.
As with many federal agency rules, CMS is requesting stakeholder feedback using requests for information focused on possibly revising Conditions of Participation to improve interoperability and electronic sharing of data by healthcare systems. CMS requests that commenters provide clear, concise proposals including data and specific examples or novel legal questions providing analysis of CMS’ authority. The deadline for such feedback is June 25, 2018.
The rule change is expected to remove excessive or redundant quality measures from a number of pay-for-performance and quality reporting programs, eliminating a large number of measures currently required of acute-care hospitals while removing duplicative measures across their five-hospital quality and value-based purchasing programs, removing 19 measures entirely and removing duplication caused by an additional 21 measures while maintaining hospital quality and patient safety. There are several other changes in the works that are aimed at reducing time spent on paperwork, allowing healthcare professionals to focus on providing better patient care.
For eligible hospitals reporting clinical quality measurements (CQMs) electronically, the reporting period will be one calendar quarter from 2019 selected by the healthcare system, with a minimum of four self-selected CQMs out of the 16 that are recorded. In 2020, eight of the 16 CQMs will be removed to create more meaningful measures. There are a number of other measures being selected for elimination, which combined with the others mentioned above total savings of 2 million burden hours and $75 million in savings.
If you’re not sure what you need to do with your EHR system to meet these new requirements, you’re not alone. Fortunately, Exscribe is here to help. Please feel free to contact us today to schedule a demo of our orthopedic EHR system to see how your practice can excel.